Andon九安品牌怎么样 申请店铺
外推网助力Andon九安品牌出海!将品牌入驻外推网,定制Andon九安品牌推广信息,可以显著提高Andon九安产品曝光,简直是跨境电商爆单神器!目前仅需1000元/年哦~
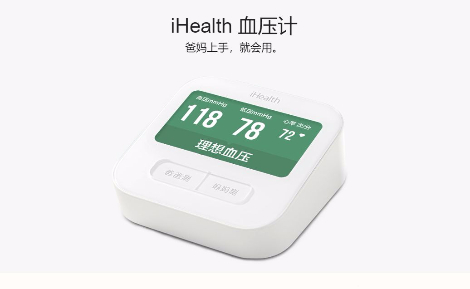
天津九安医疗电子股份有限公司成立于1995年,2007年改制为股份制公司,2010年6月10日在深圳证券交易所中小版上市。公司是集研发、生产、销售于一体的高新技术企业,主要产品为血压测量产品、血糖测试产品和其他高附加值的家用健康产品。
发展简史
1995年公司自主研发成功的KD-322型九安电子血压计,国内首创,获国家认可的CPA证书。2000年通过了ISO9001国际质量体系认证。现有123款产品获得欧盟CE认证,27款产品获得美国FDA认证,2款血压计获得英国高血压协会BHS认证。公司自主知识产权专利31项,正在申请的专利有40项。
2008年度公司电子血压计的产销总量列世界第五位。产品出口遍及100多个国家,仅德国市场所占份额达到50%。打破了由日本及台湾厂商对电子血压计行业的垄断格局,成为行业内唯一能够与其抗衡的中国内地企业。
技术研发
2007年公司技术中心被评为天津市市级企业技术中心
2008年公司被继续认定为天津市高新技术企业
公司拥有近100名技术研发人员,研发方向分为电子血压计、血糖仪、低频产品、心电监测四大类产品方向。所有产品均掌握核心技术、拥有自主知识产权技术立项横跨血压数据监测、血糖数据监测、体温数据监测、心血管系统监测等众多生理参数监测领域,以及脉冲、激光、红外、加热、生物电等保健领域
体系认证:ISO9001和ISO13485国际质量管理体系认证
产品认证:138款产品获得欧盟CE认证,68款产品通过美国FDA510(K)申请
多款血压计通过英国BHS、德国DHL、欧洲ESH认证
自主知识产权:目前拥有的自主知识产权专利34项,正在申请的专利有40余项
生产规模
公司电子血压计6条生产线,日产量达到2万台,月产能达到30万台,年产能400万台。
为了适应国际国内市场的发展,为了进一步增加国际市场份额,特别是打开美国市场,公司对内部各项管理制度和体系文件,按照美国QSR820法规进一步完善,使公司的管理更加规范化、标准化、国际化。
国内市场
九安公司在开拓国际市场的同时,大力进军国内市场,树立“九安”品牌。
公司从2003年开始深耕中国市场,五年时间里,“九安电子血压计”国内市场取得了快速、长足的发展。
从2003年至今,我公司已成功开拓了北京、天津、上海、广州4个直营市场、150个分销市场。至2008年,九安电子血压计市场占有率跃居国产品牌第一。
2009年,九安携手科里纳,共同缔造“九安血压计”品牌。加强国内消费者对九安品牌的认识,树立九安品牌在中国消费者心中的独特位置。
国际市场
九安公司在国际市场采用OEM、ODM的商业运作模式,产品出口德国、英国、法国、美国、意大利、西班牙、葡萄牙、荷兰、比利时、瑞典、挪威、丹麦、芬兰、土尔其、波兰、匈牙利、捷克、保加利亚、乌克兰、俄罗斯、澳大利亚、新西兰、加拿大、巴西、智利、哥伦比亚、巴拉圭、秘鲁、委内瑞拉、以色列、尼日利亚、印度、马来西亚、新加坡、印度尼西亚、韩国等50多个国家。
据中国海关统计,公司在2008年全球家用电子血压计及相关产品出口中,出口量升至全球排名榜第5位,是唯一进入全球行业前列的内地企业。
近年来,国际电子血压计市场正以15%~20%的速度增长,未来的前景将愈发广阔,争取两年内进入全球行业三强。
Tianjin Jiu'An Medical Electronic Co., Ltd. was founded in 1995, restructured into a joint-stock company in 2007, and listed in the small and medium-sized version of Shenzhen Stock Exchange on June 10, 2010. The company is a high-tech enterprise integrating R & D, production and sales. Its main products are blood pressure measurement products, blood glucose test products and other high added value household health products. Brief history of development: in 1995, the company independently developed and succeeded in kd-322 type Jiuan electronic sphygmomanometer, which was the first in China and obtained the CPA certificate recognized by the state. In 2000, it passed the ISO9001 international quality system certification. At present, 123 products have obtained EU CE certification, 27 products have obtained FDA certification, and 2 sphygmomanometers have obtained BHS certification. The company has 31 independent intellectual property patents and 40 patents are being applied for. In 2008, the company's total production and sales of electronic sphygmomanometers ranked fifth in the world. The products are exported to more than 100 countries, and only Germany accounts for 50% of the market. It has broken the monopoly pattern of Japanese and Taiwan manufacturers in the electronic sphygmomanometer industry and become the only mainland enterprise in the industry that can compete with it. Technology R & D in 2007, the company's technology center was rated as Tianjin municipal enterprise technology center in 2008, the company was continuously recognized as Tianjin high tech enterprise company with nearly 100 technology R & D personnel. The R & D direction is divided into four major product directions: electronic sphygmomanometer, blood glucose meter, low-frequency product and ECG monitoring. All products have core technology, independent intellectual property technology project across blood pressure data monitoring, blood glucose data monitoring, body temperature data monitoring, cardiovascular system monitoring and many other physiological parameter monitoring fields, as well as pulse, laser, infrared, heating, bioelectricity and other health care field system certification: ISO9001 and ISO13485 international quality management system certification product certification: 138 The products have obtained EU CE certification, 68 products have passed the US fda510 (k) and applied for several sphygmomanometers have passed the British BHS, German DHL and European ESH certification. At present, the company has 34 patents of independent intellectual property rights, and more than 40 patents are being applied for. The company has 6 production lines of electronic sphygmomanometers, with a daily production capacity of 20000, a monthly production capacity of 300000 and an annual production capacity of 4 million 。 In order to adapt to the development of the international and domestic market and further increase the international market share, especially to open the American market, the company further improved the internal management system and system documents in accordance with the qsr820 regulations of the United States, so as to make the company's management more standardized, standardized and international. At the same time of opening up the international market, Jiuan company has made great efforts to enter the domestic market and establish the brand of "Jiuan". Since 2003, the company has been deeply involved in the Chinese market. In five years, the domestic market of "Jiuan electronic sphygmomanometer" has made rapid and substantial development. Since 2003, our company has successfully developed 4 direct markets and 150 distribution markets in Beijing, Tianjin, Shanghai and Guangzhou. By 2008, the market share of Jiuan electronic sphygmomanometer jumped to the first place among domestic brands. In 2009, Jiuan and Corina jointly created the brand of "Jiuan sphygmomanometer". Strengthen the domestic consumers' understanding of Jiu'An brand and establish its unique position in the hearts of Chinese consumers. International market nine an company adopts the business operation mode of OEM and ODM in the international market, and its products are exported to Germany, Britain, France, the United States, Italy, Spain, Portugal, Holland, Belgium, Sweden, Norway, Denmark, Finland, Turkey, Poland, Hungary, Czech, Bulgaria, Ukraine, Russia, Australia, New Zealand, Canada, Brazil, etc Chile, Colombia, Paraguay, Peru, Venezuela, Israel, Nigeria, India, Malaysia, Singapore, Indonesia, South Korea and other 50 countries. According to the statistics of China Customs, in 2008, the export volume of the global household electronic sphygmomanometer and related products rose to the fifth place in the world, and the company is the only mainland enterprise that has entered the forefront of the global industry. In recent years, the international electronic sphygmomanometer market is growing at a rate of 15% - 20%, and the future prospects will be more broad, striving to enter the top three global industries within two years.
本文链接: https://brand.waitui.com/c3570b7a0.html 联系电话:请联系客服添加 联系邮箱:请联系客服添加

















 浙公网安备 33011802001999号
浙公网安备 33011802001999号
